Article Types Considered
To facilitate manuscript submission, a mandatory docx-file template is available for each article type. Please follow the corresponding links. Highlighted instructions in the template must be removed before submission. Submit your manuscript in Word format; do not save it as a PDF. Please note that a separate cover letter is no longer required.
Go to the Submissions page to make a submission.
1-Empirical research articles
Empirical research articles disseminate the results of studies based on a variety of designs that apply qualitative, quantitative or mixed methods and may refer to different conceptual frameworks in order to address specific research questions and hypotheses in an appropriate and coherent manner.
For mixed-method research
MMAT (Mixed Methods Appraisal Tool): A tool specially designed to assess the quality of mixed-method research. It allows one to evaluate the qualitative and quantitative components of the studies.
- Articles concerning the development or validation of an instrument of measure must, when initially submitted, be accompanied by the complete instrument in a separate or supplementary file. Moreover, articles regarding an instrument of measure must include an empirical evaluation (data collection and analysis) documenting the instrument’s validity or reliability.
- Authors who submit a manuscript of clinical trial evaluating the effects of one or more health-related interventions on health outcomes of participants (Social Sciences and Humanities Research Council [SSHRC], Natural Sciences and Engineering Research Council [NSERC] of Canada, Canadian Institutes of Health Research [CIHR], 2018) must have registered the study protocol prior to the recruitment of the first participant in a publicly accessible registry (see ISRCTN or the WHO International Clinical Trials Registry Platform: https://www.who.int/clinical-trials-registry-platform) in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans and the criteria of the International Committee of Medical Journal Editors. Registration is required for the manuscript to be considered for the double-blind peer review. The registry name and the study protocol registration number must be provided when initially submitting the manuscript. In addition, all clinical trial manuscripts must be accompanied, at the time of submission, by the 25-item CONSORT checklist completed.
Clinical trials include the evaluation of interventions. As defined by the Tri-Council Policy of Canada: “An intervention is the planned imposition of a condition or set of conditions on participants for the purposes of research. The conditions may be such things as a task, an activity, a treatment, exposure to stimuli, or a change to the environment. The purpose of the research may be to describe, measure, evaluate, explain, or observe participants’ reactions or responses to one or more of the imposed conditions” (SSHRC, NSERC, CIHR, pp. 144). The journal will consider clinical trial manuscripts that meet criteria for ethics, registration, and scientific quality.
Empirical research articles should be 3000–5000 words long (excluding endnotes, references, tables and figures).
Authors who want to submit an empirical research article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/28
2-Knowledge synthesis articles
The journal accepts knowledge synthesis articles that present transparent, explicit and rigorous methods. These syntheses approach aim to produce new knowledge by responding to a research question; follow detailed and reproducible protocols relatively to the type of synthesis, search strategy, eligibility criteria, data collection methods and tools, and analysis performed. This excludes exploratory reviews of narrative type or traditional literature reviews. The journal no longer accepts this type of reviews since September 2023.
For mixed-method research
PRISMA adapted for Mixed Methods: Although PRISMA (https://www.prisma-statement.org/prisma-2020) is not specifically designed for literature reviews of mixed methods studies, some research teams have adapted its principles for such reviews.
Table 1a
Examples of knowledge synthesis accepted by the journal and associated reporting standards
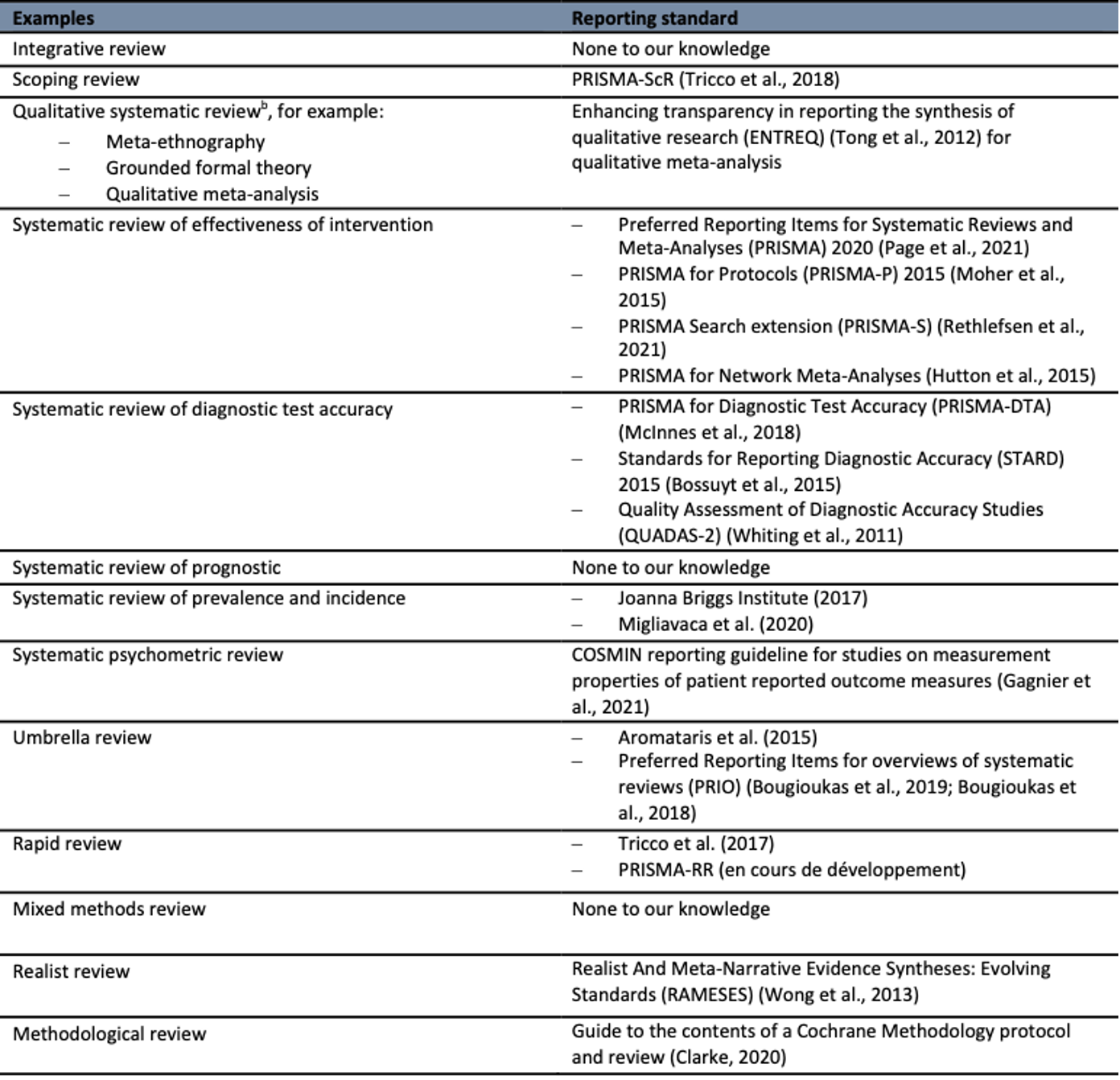
Notes. a Adapted from : Fontaine, G., Maheu-Cadotte, M-A., Lavallée, A., Mailhot, T., Lavoie, P., Rouleau, G., Vinette, B., Ramírez García, M-P. et Bourbonnais, A. (2022). Designing, planning, and conducting systematic reviews and other knowledge syntheses: Six key practical recommendations to improve feasibility and efficiency. Worldviews on Evidence-Based Nursing, 19(6), 434–441. https://doi.org/10.1111/wvn.12609
b Thorne, Sally. (2022). Qualitative meta-synthesis. Nurse Author & Editor, 32(1), 15-18. https://doi.org/10.1111/nae2.12036
Knowledge synthesis articles should be 3000–5000 words long (excluding endnotes, references, tables and figures).
Authors who want to submit a knowledge synthesis article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/28
3-Theoretical discussion and methodology articles
Theoretical discussion and methodology articles include:
1) Articles that aim at proposing methodological innovations to overcome challenges or gaps concerning methods or theories by examining different facets of the process and procedures of research. For example:
• Populations, sampling and recruitment methods
• Data collection, management and analysis methods
• Participatory research methods
• Epistemology, paradigms and research methods
• Health intervention evaluation methods.
2) Articles that report the development of interventions to improve health. When developing interventions, researchers use different approaches (e.g. collaborative process, community engagement), theories (e.g. Intervention Mapping, theory of change) and methods (e.g. mixed methods, qualitative, formative) to design interventions targeting different health experiences (e.g. behaviors, comorbidities, chronic diseases) of key populations (see the review by Croot et al. 2019: https://pilotfeasibilitystudies.biomedcentral.com/articles/10.1186/s40814-019-0512-8#Tab1).
Theoretical discussion and methodology articles may be prepared and submitted as a long article (e.g., lessons learned of a primary study) of 3000–5000 words or as a brief report (e.g., critical discussion centred exclusively on the use of a conceptual framework) of 1500–3000 words (excluding endnotes, references, tables and figures).
Authors who want to submit a theoretical discussion and methodology article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/30
4-Knowledge-to-action articles
knowledge-to-action articles document how the use of new knowledge into action (“encompasses the use of knowledge by practitioners, policymakers, patients, and the public” (p. 14): Graham, I. D., Logan, J., Harrison, M. B., Straus, S. E., Tetroe, J., Caswell, W., & Robinson, N. (2006). Lost in knowledge translation: time for a map?. The Journal of continuing education in the health professions, 26(1), 13–24. https://doi.org/10.1002/chp.47) can be highly relevant and rigorous. For example, an evaluation process that measures the impacts of implementing knowledge into routine practice on indicators of health, well-being and functioning. This body of works involves all stakeholders concerned with improving actions. An example of this type of published article:
- Demers-Bouchard, R., Gallani, M. C., & Balg, C. (2025). Planification de l’implantation du journal de bord comme stratégie d’humanisation aux soins intensifs adultes. Science infirmière et Pratiques en Santé, 8(1), 116–144. https://doi.org/10.62212/snahp.153
For mixed-method research
TDR’s Guide to Mixed Methods: This guide provides guidance for planning and conducting research using both qualitative and quantitative methods in the same project.
knowledge-to-action articles may be prepared and submitted as a long article of 3000–5000 words or as a brief report of 1500–3000 words (excluding endnotes, references, tables and figures).
Authors who want to submit a knowledge-to-action article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/32
5-Research protocol articles
Definition
A research protocol article is a brief report (2,500–3,000 words) that outlines all the components and steps of empirical research or knowledge synthesis (see above the types of knowledge synthesis accepted by the journal) in its initial or developmental phase, providing sufficient detail on the research problem, methods used and anticipated issues.
Objectives and scope
The purpose of publishing protocols in the journal Science of Nursing and Health Practices / Science infirmière et pratiques en santé is to:
- Reduce publication bias or selective reporting of results,
- Rapidly disseminate information on ongoing studies to clinicians, researchers, decision makers and the public,
- Promote collaboration between research teams,
- Reduce the duplication of studies,
- Provide greater detail than the study registration in cases where a study must also be registered.
Criteria for submitting protocol manuscripts
- Manuscripts will be assessed in the first instance according to the following criteria:
- The protocol must address an original research topic and/or new research method and be closely related to health practices (objectives and scope of the journal).
- The protocol must meet the quality criteria for publication (see https://journals.plos.org/plosone/s/criteria-for-publication; https://www.osjournal.org/criteria_for_publication.html), regardless of its approval by a scientific/ethics committee or support from a funding agency. The editorial team may reject, in the first instance, any protocol that does not meet these quality of publication criteria.
- Authors must follow the SPIRIT guidelines in submitting a clinical trial protocol or randomized study, or any other guideline appropriate to their research design housed on the EQUATOR
- For a knowledge synthesis protocol, the authors present an appendix with tables showing data extraction anticipated following the guidelines of the JBI Manual for Evidence Synthesis of the Joanna Briggs Institute: https://jbi-global-wiki.refined.site/space/MANUAL
- The written language must be of high quality to expedite the protocol evaluation process and avoid linguistic revisions.
- Publication of the protocol does not replace registration of the study in a recognized database (ISRCTN, ICTRP, ClinicalTrials.gov, PROSPERO); all studies involving evaluations of health outcomes and systematic reviews must first be registered. Authors must include the registry name and study protocol registration number when they first submit their manuscript. The protocol article should provide more details and clarifications than the registration in terms of the research problem, the methods used and the anticipated issues.
- The protocol must be submitted to the journal Science of Nursing and Health Practices / Science infirmière et pratiques en santé after its approval by a human research ethics committee and before data collection is completed.
- Once these criteria have been met, the manuscript will be submitted for double-blind peer review based on scientific quality criteria.
- Authors whose manuscripts are accepted/published are invited to submit the manuscript of the study results once the study is completed.
For the presentation of research protocol articles, manuscripts must be written in the future tense, while certain parts, such as the literature review, can be written in the past tense, if they refer to what was carried out to guide the protocol proposal. However, the data collection must be written in the future tense.
Authors who want to submit a research protocol article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/34
6-Short articles
The short article presents, in a concise manner, research findings or data that do not warrant a full article. Examples include pilot studies, preliminary analysis of results with a limited sample, exploratory studies that are not yet sufficiently substantiated to warrant a full article, or additional data from a broader study, such as a secondary analysis.
- 1500 words maximum.
- We expect a maximum of 5 authors and 10 references.
- No abstract and no keywords.
- The publishing ethics of the journal apply; therefore, a statement of conflict of interest is required.
- Short papers will be reviewed by the editorial team for scientific quality and quality of written style, including the use of epicene language and a respectful tone.
- A double anonymous review applies.
- The editorial team reserves the right to decline a short paper based on the above-mentioned criteria.
Authors who want to submit a short article must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/36
Letters to the Editor
The editorial team of the journal is committed to fostering scientific discussion and scholarly exchange. Therefore, we invite readers to submit:
1) Letters addressing recent content published by the journal, or to;
2) propose new topics for discussion in the form of well-founded comments that align with the journal’s objectives and scope.
- 1500 words maximum.
- We expect a maximum of 5 authors and 10 references.
- No abstract and no keywords.
- The publishing ethics of the journal apply to the Letters to the Editor, therefore a statement of conflict of interest is required.
- Letters to the Editor will be reviewed by the editorial team for scientific quality and quality of written style, including the use of epicene language and a respectful tone.
- A double anonymous review of the scientific content applies.
- The editorial team could ask authors to revise their Letter and reserve the right to decline a Letter based on the above-mentioned criteria.
For 1) comments on a recently published article to stimulate scientific discussion:
- In order to encourage exchanges in a relatively short period of time, we ask that the Letter be submitted within three months of the publication of the article on the journal’s website in the “Forthcoming” section (https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/forthcoming).
- Once accepted, the Letter will be immediately published in this section and forwarded to the authors.
- Authors may respond to the comment in a Letter to the Editor format if they wish to; this response will undergo the same review as the comment.
- Authors of the initial comment will not have the opportunity to respond to the response of authors.
Authors who want to submit a Letter to the Editor must use the following template: https://sips-snahp.ojs.umontreal.ca/index.php/sips-snahp/libraryFiles/downloadPublic/38
